Explain Differences in Solubility Based on Imfs
Acid-Base Solubility Equilibrium chemistry b- moles packet name. When most liquids are cooled they eventually freeze and form crystalline solids solids in which the atoms ions or molecules are arranged in a definite repeating pattern.
Physical properties commonly discussed when relating to IMFs in pure substances are.
. In each LESSON above youll find a tutorial quiz key exam question. General Equilibrium Unit 9. Explain the ways in which crystal defects can occur in a solid.
The questions are often based on the Large Data Set but you can still answer them even if you know nothing about the LDS. Acid-Base Equilibrium Unit 10. The CORRELATION REGRESSION REVISION PACK has all the exam questions youll need to.
Chemistry EssentialsStoichiometry Unit 2. Atomic Structure Periodicity Unit 5. Explain the relation between the intermolecular forces present within a substance and the temperatures associated with changes in its physical state.
Be sure to read the linked answer to review if you are unfamiliar with IMFs. Theres more interesting things about correlation in the second year. IMFs are the various forces of attraction that may exist.
_____ page 8 chemistry. States of Matter IMFs Solutions Unit 7. The phase in which a substance exists depends on the relative extents of its intermolecular forces IMFs and the kinetic energies KE of its molecules.
As was the case for gaseous substances the kinetic molecular theory may be used to explain the behavior of solids and. The resulting materials are called. Gas Laws Unit 4.
Melting and boiling points - when molecules go from. Reactions Aqueous Solutions Unit 3. As mentioned here intermolecular forces IMFs are important because they are the leading cause for differences in physical properties between similar molecules.
The differences in the properties of a solid liquid or gas reflect the strengths of the attractive forces between the atoms molecules or ions that make up each phase. Identify the types of intermolecular forces experienced by specific molecules based on their structures. It is also possible for a liquid to freeze before its molecules become arranged in an orderly pattern.

4 4 Solubility And Intermolecular Forces Sl Youtube
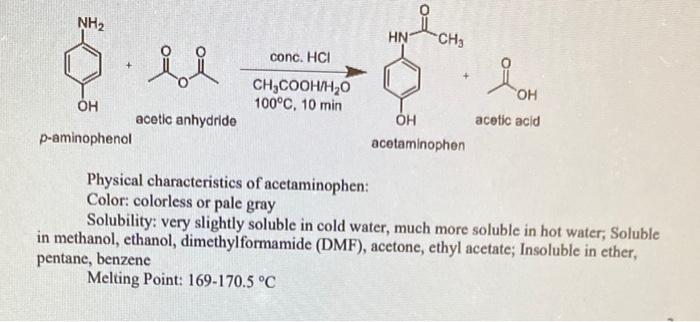
Solved 2 In Lab 2 You Will Be Testing The Solubility Of Chegg Com

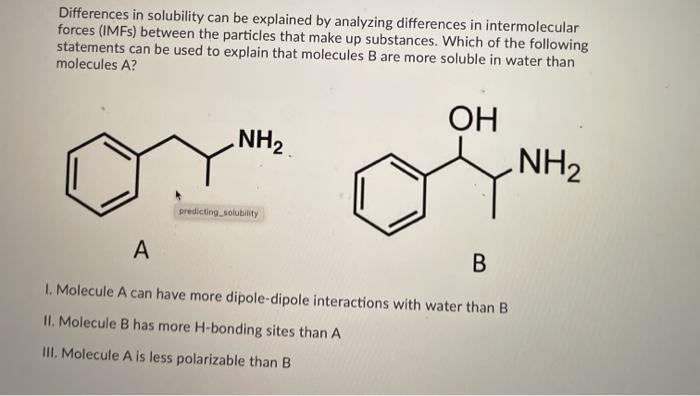
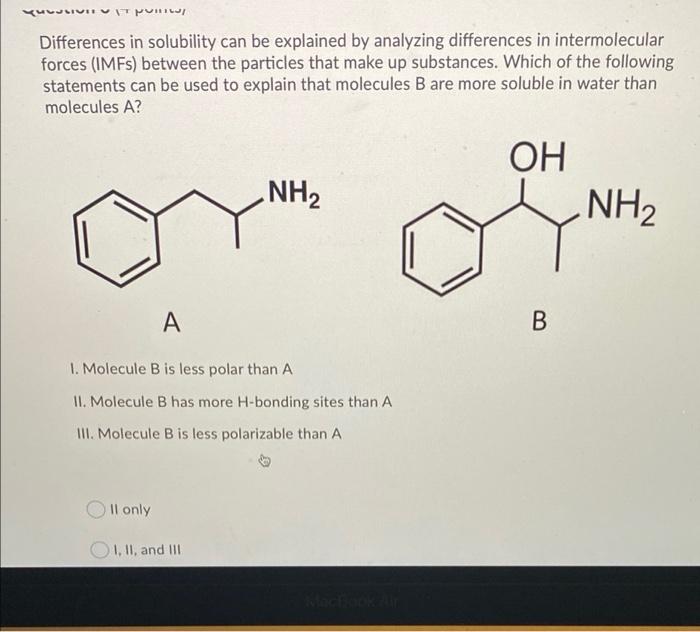
Solved Differences In Solubility Can Be Explained By Chegg Com
0 Response to "Explain Differences in Solubility Based on Imfs"
Post a Comment